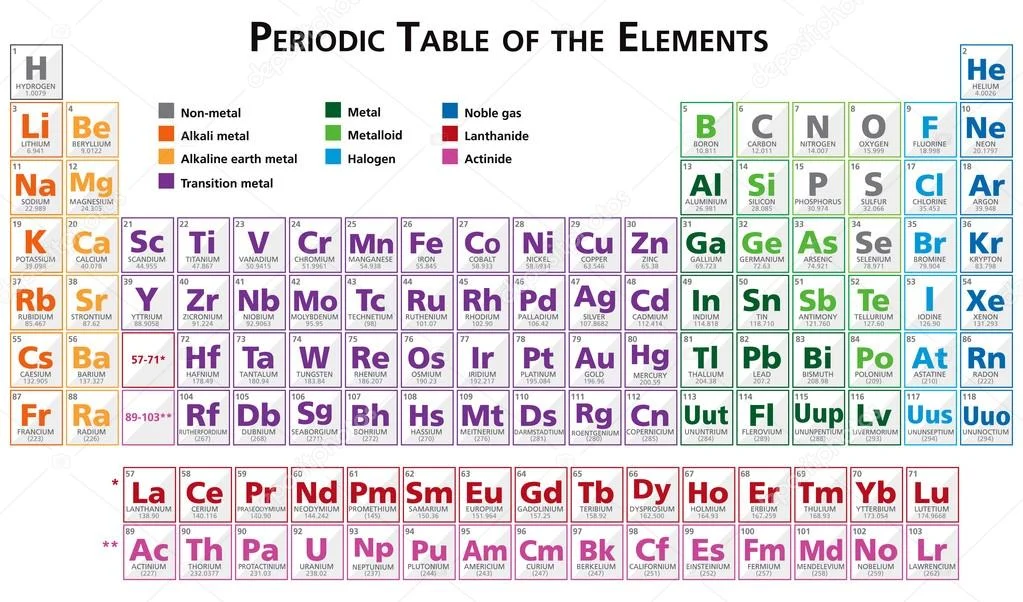
What is the Periodic Table of Elements?
The Periodic Table is one of the most significant achievements in science, providing a systematic framework for understanding all known elements. This elegant chart organizes elements based on their atomic structure and recurring chemical properties, serving as an indispensable tool for students and scientists worldwide. How did the periodic table originate? The modern Periodic Table evolved from early 19th-century attempts to classify elements, culminating in Dmitri Mendeleev’s 1869 masterpiece that successfully predicted properties of undiscovered elements. Today’s table arranges elements by increasing atomic number, revealing profound patterns in how matter behaves at its most fundamental level.
The table’s design reflects the quantum mechanical nature of atoms, where electron configurations determine chemical behavior. Each position in the table tells a story about an element’s characteristics, from its reactivity to the types of chemical bonds it forms. The Periodic Table isn’t just a static reference chart; it’s a dynamic map that continues to expand as new elements are synthesized in laboratories. Its universal adoption across scientific disciplines demonstrates its fundamental importance in our understanding of the physical world, from simple chemical reactions to advanced materials science.
Atomic Structure and Organization Principles
The Periodic Table’s organization stems from the quantum mechanical model of the atom. Each element is defined by its atomic number – the number of protons in its nucleus – which determines its position in the table. The table’s vertical columns, called groups, contain elements with similar electron configurations in their outermost shell, leading to comparable chemical properties. What determines an element’s chemical behavior? The arrangement of electrons, particularly valence electrons in the outermost shell, primarily dictates how an element will interact with others to form molecules and compounds.
Horizontal rows, known as periods, represent elements with the same number of electron shells. As we move across a period, atomic radius decreases while electronegativity generally increases, creating predictable trends in reactivity. The table also divides elements into blocks (s, p, d, f) based on which atomic orbitals their valence electrons occupy. This sophisticated organization allows chemists to predict everything from an element’s preferred oxidation states to the types of chemical reactions it will undergo, making the Periodic Table an essential tool for understanding and manipulating matter.
Major Groups and Their Characteristics
The Periodic Table’s groups reveal fascinating patterns in chemical behavior. Group 1 contains alkali metals like lithium and sodium – highly reactive elements that readily lose one electron to form positive ions. Group 17 comprises halogens like fluorine and chlorine, which are equally reactive in the opposite direction, tending to gain electrons to complete their valence shells. Why are noble gases so unreactive? Group 18 noble gases like neon and argon have complete valence electron shells, making them exceptionally stable and largely inert under normal conditions.
Transition metals in the d-block exhibit variable oxidation states and often form colorful compounds with catalytic properties. The lanthanides and actinides, placed separately below the main table, include elements crucial for modern technology, from neodymium in magnets to uranium in nuclear power. Understanding these group trends enables scientists to select appropriate elements for specific applications, whether developing new catalysts for industrial processes or designing pharmaceutical compounds with precise biological activity.
Chemical Periodicity and Predictive Power
The term “periodic” in Periodic Table refers to the repeating patterns of chemical and physical properties that occur at regular intervals. These periodic trends include atomic radius, ionization energy, electron affinity, and electronegativity, all of which follow predictable patterns across periods and down groups. How does atomic radius change across the periodic table? Atomic radius decreases moving left to right across a period due to increasing nuclear charge, but increases down a group as additional electron shells are added.
The table’s true power lies in its predictive capability. Mendeleev famously left gaps for undiscovered elements and accurately predicted their properties. Today, chemists can anticipate how unfamiliar elements will behave based on their position in the table. This predictive power extends to understanding complex chemical reactions, material properties, and even the behavior of elements under extreme conditions. The Periodic Table thus serves not just as a catalog of known elements, but as a guide to chemical behavior throughout the universe.
The Periodic Table in Modern Science and Technology
Beyond academic study, the Periodic Table plays crucial roles across scientific and technological fields. In materials science, understanding periodic trends helps engineers design alloys with specific properties, from lightweight aluminum compounds for aerospace to shape-memory alloys for medical devices. How does the periodic table guide materials design? Scientists use position in the table to predict how elements will interact, enabling deliberate creation of materials with desired conductivity, strength, or reactivity.
The table informs environmental science through understanding elemental cycles and pollutant behavior. In pharmacology, drug design leverages knowledge of how different elements and their compounds interact with biological systems. Nuclear science relies heavily on the table, with fission and fusion reactions involving specific elements and their isotopes. Even emerging technologies like quantum computing look to the Periodic Table for elements with suitable quantum properties. As research continues, the table remains central to innovation across disciplines.
Table: Periodic Trends and Their Patterns
| Trend | Pattern Across Period (left to right) | Pattern Down Group (top to bottom) |
|---|---|---|
| Atomic Radius | Decreases | Increases |
| Ionization Energy | Increases | Decreases |
| Electronegativity | Increases | Decreases |
| Metallic Character | Decreases | Increases |
| Electron Affinity | Generally increases | Generally decreases |
Elements in Biological Systems and Environment
The Periodic Table reveals fascinating patterns in biological importance. Only about 25-30 elements are essential to life, with carbon, hydrogen, oxygen, and nitrogen forming the basis of organic molecules like proteins, DNA, and carbohydrates. Why is carbon so fundamental to life? Carbon’s unique ability to form four stable bonds allows creation of complex molecules necessary for biological functions, making it the backbone of all known life forms.
Metals play crucial biological roles too – iron in hemoglobin transports oxygen, while zinc and copper serve as cofactors for enzymes. The table also helps us understand environmental processes: carbon and nitrogen cycles, heavy metal toxicity, and nutrient availability in ecosystems. Studying how elements move through biological and environmental systems – their biogeochemical cycles – relies fundamentally on understanding their chemical properties as revealed by their periodic table positions.
The Future of the Periodic Table
The Periodal Table continues to evolve as scientific knowledge advances. New elements are synthesized in particle accelerators, extending the table to higher atomic numbers. These superheavy elements push the boundaries of nuclear stability and test predictions of quantum mechanical models. What challenges exist in discovering new elements? Creating and identifying new elements becomes increasingly difficult as they become more unstable, often existing for only fractions of a second before decaying.
Researchers also explore the “island of stability,” a theoretical region where superheavy elements might exhibit unusual stability. Beyond synthetic elements, scientists continue discovering new aspects of familiar elements, from novel allotropes like graphene (carbon) to unexpected compounds like sodium chloride under high pressure. The table may even need reimagining as we discover more about elements under extreme conditions or potentially in extraterrestrial environments. Despite its age, the Periodic Table remains a vibrant, growing scientific tool.
Frequently Asked Questions (FAQ)
1. How are elements organized in the periodic table?
Elements are arranged by increasing atomic number (number of protons) in rows called periods, and grouped into columns based on similar electron configurations and chemical properties.
2. What do the periods and groups represent?
Periods represent elements with the same number of electron shells, while groups contain elements with the same number of valence electrons, leading to similar chemical behavior.
3. Why are some elements more reactive than others?
Reactivity depends largely on how easily an element gains or loses electrons to achieve a stable electron configuration, which is determined by its position in the periodic table.
4. How many elements occur naturally?
Of the 118 known elements, 94 occur naturally on Earth, while elements 95-118 have been synthesized in laboratories through nuclear reactions.
5. Can the periodic table predict new elements?
Yes, the periodic table’s patterns allow scientists to predict properties of undiscovered elements, though synthesizing and confirming these elements requires sophisticated technology.
Keywords: Periodic Table, Elements, Atom, Compound, Chemical Reaction, Chemistry, Molecule, Oxidation, Catalyst, Energy, Quantum Mechanics, Proton, Electron, Property, Metal
Tags: #PeriodicTable #Chemistry #Elements #Science #Education #STEM #Atoms #ChemicalReactions #QuantumMechanics #MaterialsScience